Adsorption of polyelectrolytes to like-charged substrates induced by multivalent counterions as exemplified by poly(styrene sulfonate) and silica†
Abstract
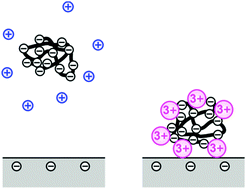
The present study demonstrates that multivalent counterions trigger adsorption of polyelectrolytes on a like-charged substrate. In particular, adsorption of polystyrene sulfonate on silica is studied experimentally in NaCl, MgCl2, and LaCl3 solutions by optical reflectivity. While adsorption is negligible in the presence of Na+, the polyelectrolyte adsorbs in the presence of Mg2+ and La3+. The adsorbed amount of the polyelectrolyte goes through a maximum as a function of the salt concentration. This maximum increases with increasing valence and shifts to lower salt concentrations. At low salt concentration, the adsorption is negligible. At intermediate salt level, ripening and multilayer formation leads to continuous growth of the adsorbed layer. At higher salt level, blocking and formation of a monolayer lead to saturation. These results are tentatively interpreted in terms of a charge reversal of the polyelectrolyte–metal complex. The molecular mass of the polyelectrolyte has an important effect on the adsorption behavior, whereby the tendency towards ripening becomes more pronounced at large molecular mass.

 Please wait while we load your content...
Please wait while we load your content...