Geometries, stabilities and fragmental channels of neutral and charged sulfur clusters: SnQ (n = 3–20, Q = 0, ±1)†
Abstract
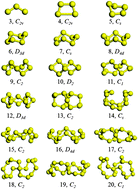
We have performed unbiased searches for the global minimum structures of neutral and charged sulfur clusters SnQ (n = 3–20, Q = 0, ±1) relying on the CALYPSO structure searching method combined with density functional theory geometric optimization. Very accurate ab initio calculations are used to determine relative stabilities and energy ranking among competing low-lying isomers of the neutral and charged sulfur clusters obtained from the structure search. The harmonic vibrational analysis is also undertaken to assure that the optimized geometries are the true minima. It is shown that the most equilibrium geometries of sulfur clusters are closed three-dimensional (3D) helical rings, which is in agreement with the experimental observations. The binding energies, second-order energy differences, and highest occupied–lowest unoccupied molecular orbital (HOMO–LUMO) gaps of the considered species are calculated and analyzed systematically. Additionally, the fragmentation channels are determined and the results indicate that the SnQ → S2 + Sn−2Q channel is a route that the small clusters (n = 3–10) favor, while the larger species (n = 13–20) prefer the SnQ → S8 + Sn−8Q channel.

 Please wait while we load your content...
Please wait while we load your content...