Structure determination of bound nitrogen-based adducts with copper(ii) acetylacetonato; an EPR, ENDOR and DFT study†
Abstract
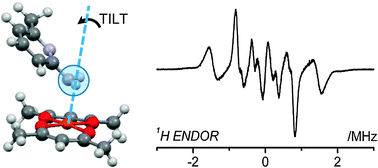
The adducts of bis(acetylacetonato)–copper(II), [Cu(acac)2], formed with a range of nitrogen heterocycles including pyridine (2), methylpyridines (3,4,5), amino-methylpyridines (6,7) and diazines (8,9,10) were investigated in frozen solution using X-band EPR and 1H ENDOR spectroscopy. The small perturbations to the EPR spin Hamiltonian parameters (g and CuA) were consistent with the axial coordination of the nitrogen bases to Cu(II), and found to be dependent on both the basicity and steric influence of the coordinating substrate. The detailed structure of two adducts was then investigated by angular selective 1H ENDOR and DFT. For the [Cu(acac)2](pyridine) adduct, axial coordination of the substrate was found to occur via the pyridine nitrogen as expected, producing a characteristic 1H hyperfine coupling (HAi = −2.6, −2.04, 4.7 MHz; β = 36°; aiso = 0.2 MHz) arising from the ortho-1H in the ring 2 or 6 position. These results were confirmed by DFT. However, in the [Cu(acac)2](2-amino-6-methyl-pyridine) adduct, the ENDOR data revealed a substantially different 1H hyperfine coupling (HAi = −4.52, −3.35, 6.47 MHz; β = 14°; aiso = −0.47 MHz) arising from the –NH2 amino protons. Analysis of this experimentally derived tensor in conjunction with the calculated DFT tensors, revealed that the 2-amino-6-methyl-pyridine substrate binds to Cu(II) via the exocyclic amino pyridine nitrogen, but with a tilt angle of 20° of the pyridine ring away from the geometry optimised structure. These results reveal how important structural information on the coordination geometry of Cu(II) adducts can be obtained by 1H ENDOR, but only when the complete angular dependency profile of the ENDOR data is thoroughly considered.

 Please wait while we load your content...
Please wait while we load your content...