Unusual denaturation trajectory of bovine gamma globulin studied by fluorescence correlation spectroscopy†
Abstract
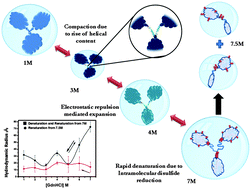
Non-native and denatured states of proteins have received increasing attention because of their relevance to issues such as protein folding and stability. In this context, the pathway of polypeptide collapse and random coil formation in a denatured protein is a subject of much interest. Most proteins so far studied have shown monotonic expansion of their hydrodynamic radius (RH) in the presence of increasing concentration of chaotropes. We have studied GdnHCl-induced folding transitions and conformational states of a multi-domain protein, bovine gamma globulin, using fluorescence, circular dichroism and fluorescence correlation spectroscopy (FCS). FCS measurements showed that for gamma globulin, contrary to the observed trend, RH decreases with increasing GdnHCl concentration up to 3 M. At higher GdnHCl concentration, RH starts to increase but exhibits complicated behavior in the form of two sharp maxima at 4 M and 7 M. Further experiments suggest that the maximum at 4 M GdnHCl arises due to electrostatic interaction, whereas the one at 7 M GdnHCl corresponds to the usual expanded conformation due to denaturation. Beyond 7 M GdnHCl, RH decreases drastically and is shown to result from fragmentation of the protein caused by rupture of disulphide bonds by the high GdnHCl concentration. Our results demonstrate the capability of FCS in revealing intricate details of the unfolding trajectory that eludes conventional ensemble techniques such as fluorescence and CD.

 Please wait while we load your content...
Please wait while we load your content...