Electrochemical in situ investigations of SEI and dendrite formation on the lithium metal anode
Abstract
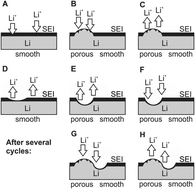
This comparative work studies the self-enforcing heterogeneity of lithium deposition and dissolution as the cause for dendrite formation on the lithium metal anode in various liquid organic solvent based electrolytes. In addition, the ongoing lithium corrosion, its rate and thus the passivating quality of the SEI are investigated in self-discharge measurements. The behavior of the lithium anode is characterized in two carbonate-based standard electrolytes, 1 M LiPF6 in EC/DEC (3 : 7) and 1 M LiPF6 in EC/DMC (1 : 1), and in two alternative electrolytes 1 M LiPF6 in TEGDME and 1 M LiTFSI in DMSO, which have been proposed in the literature as promising electrolytes for lithium metal batteries, more specifically for lithium/air batteries. As a result, electrolyte decomposition, SEI and dendrite formation at the lithium electrode as well as their mutual influences are understood in the development of overpotentials, surface resistances and lithium electrode surface morphologies in subsequent lithium deposition and dissolution processes. A general model of different stages of these processes could be elaborated.

 Please wait while we load your content...
Please wait while we load your content...