Control of selectivity in allylic alcohol oxidation on gold surfaces: the role of oxygen adatoms and hydroxyl species†
Abstract
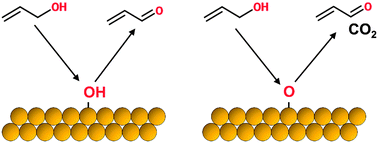
Gold catalysts display high activity and good selectivity for partial oxidation of a number of alcohol species. In this work, we discuss the effects of oxygen adatoms and surface hydroxyls on the selectivity for oxidation of allylic alcohols (allyl alcohol and crotyl alcohol) on gold surfaces. Utilizing temperature programmed desorption (TPD), reactive molecular beam scattering (RMBS), and density functional theory (DFT) techniques, we provide evidence to suggest that the selectivity displayed towards partial oxidation versus combustion pathways is dependent on the type of oxidant species present on the gold surface. TPD and RMBS results suggest that surface hydroxyls promote partial oxidation of allylic alcohols to their corresponding aldehydes with very high selectivity, while oxygen adatoms promote both partial oxidation and combustion pathways. DFT calculations indicate that oxygen adatoms can react with acrolein to promote the formation of a bidentate surface intermediate, similar to structures that have been shown to decompose to generate combustion products over other transition metal surfaces. Surface hydroxyls do not readily promote such a process. Our results help explain phenomena observed in previous studies and may prove useful in the design of future catalysts for partial oxidation of alcohols.

 Please wait while we load your content...
Please wait while we load your content...