ReaxFF molecular dynamics simulations on lithiated sulfur cathode materials†
Abstract
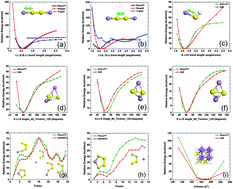
Sulfur is a very promising cathode material for rechargeable energy storage devices. However, sulfur cathodes undergo a noticeable volume variation upon cycling, which induces mechanical stress. In spite of intensive investigation of the electrochemical behavior of the lithiated sulfur compounds, their mechanical properties are not very well understood. In order to fill this gap, we developed a ReaxFF interatomic potential to describe Li–S interactions and performed molecular dynamics (MD) simulations to study the structural, mechanical, and kinetic behavior of the amorphous lithiated sulfur (a-LixS) compounds. We examined the effect of lithiation on material properties such as ultimate strength, yield strength, and Young's modulus. Our results suggest that with increasing lithium content, the strength of lithiated sulfur compounds improves, although this increment is not linear with lithiation. The diffusion coefficients of both lithium and sulfur were computed for the a-LixS system at various stages of Li-loading. A grand canonical Monte Carlo (GCMC) scheme was used to calculate the open circuit voltage profile during cell discharge. The Li–S binary phase diagram was constructed using genetic algorithm based tools. Overall, these simulation results provide insight into the behavior of sulfur based cathode materials that are needed for developing lithium–sulfur batteries.

 Please wait while we load your content...
Please wait while we load your content...