Compressibility, thermal expansion coefficient and heat capacity of CH4 and CO2 hydrate mixtures using molecular dynamics simulations†
Abstract
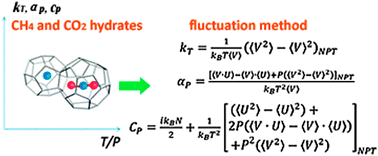
Understanding the thermal and mechanical properties of CH4 and CO2 hydrates is essential for the replacement of CH4 with CO2 in natural hydrate deposits as well as for CO2 sequestration and storage. In this work, we present isothermal compressibility, isobaric thermal expansion coefficient and specific heat capacity of fully occupied single-crystal sI-CH4 hydrates, CO2 hydrates and hydrates of their mixture using molecular dynamics simulations. Eight rigid/nonpolarisable water interaction models and three CH4 and CO2 interaction potentials were selected to examine the atomic interactions in the sI hydrate structure. The TIP4P/2005 water model combined with the DACNIS united-atom CH4 potential and TraPPE CO2 rigid potential were found to be suitable molecular interaction models. Using these molecular models, the results indicate that both the lattice parameters and the compressibility of the sI hydrates agree with those from experimental measurements. The calculated bulk modulus for any mixture ratio of CH4 and CO2 hydrates varies between 8.5 GPa and 10.4 GPa at 271.15 K between 10 and 100 MPa. The calculated thermal expansion and specific heat capacities of CH4 hydrates are also comparable with experimental values above approximately 260 K. The compressibility and expansion coefficient of guest gas mixture hydrates increase with an increasing ratio of CO2-to-CH4, while the bulk modulus and specific heat capacity exhibit the opposite trend. The presented results for the specific heat capacities of 2220–2699.0 J kg−1 K−1 for any mixture ratio of CH4 and CO2 hydrates are the first reported so far. These computational results provide a useful database for practical natural gas recovery from CH4 hydrates in deep oceans where CO2 is considered to replace CH4, as well as for phase equilibrium and mechanical stability of gas hydrate-bearing sediments. The computational schemes also provide an appropriate balance between computational accuracy and cost for predicting mechanical and thermal properties of gas hydrates in the high temperature range (≥260 K), and the schemes may be useful for the study of other complex hydrate systems.

 Please wait while we load your content...
Please wait while we load your content...