The role of an active site Mg2+ in HDV ribozyme self-cleavage: insights from QM/MM calculations†
Abstract
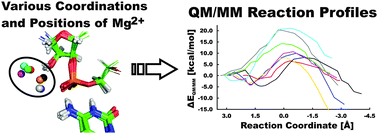
The hepatitis delta virus (HDV) ribozyme is a catalytic RNA motif embedded in the human pathogenic HDV RNA. It catalyzes self-cleavage of its sugar-phosphate backbone with direct participation of the active site cytosine C75. Biochemical and structural data support a general acid role of C75. Here, we used hybrid quantum mechanical/molecular mechanical (QM/MM) calculations to probe the reaction mechanism and changes in Gibbs energy along the ribozyme's reaction pathway with an N3-protonated C75H+ in the active site, which acts as the general acid, and a partially hydrated Mg2+ ion with one deprotonated, inner-shell coordinated water molecule that acts as the general base. We followed eight reaction paths with a distinct position and coordination of the catalytically important active site Mg2+ ion. For six of them, we observed feasible activation barriers ranging from 14.2 to 21.9 kcal mol−1, indicating that the specific position of the Mg2+ ion in the active site is predicted to strongly affect the kinetics of self-cleavage. The deprotonation of the U-1(2′-OH) nucleophile and the nucleophilic attack of the resulting U-1(2′-O−) on the scissile phosphodiester are found to be separate steps, as deprotonation precedes the nucleophilic attack. This sequential mechanism of the HDV ribozyme differs from the concerted nucleophilic activation and attack suggested for the hairpin ribozyme. We estimate the pKa of the U-1(2′-OH) group to range from 8.8 to 11.2, suggesting that it is lowered by several units from that of a free ribose, comparable to and most likely smaller than the pKa of the solvated active site Mg2+ ion. Our results thus support the notion that the structure of the HDV ribozyme, and particularly the positioning of the active site Mg2+ ion, facilitate deprotonation and activation of the 2′-OH nucleophile.

 Please wait while we load your content...
Please wait while we load your content...