Pressure-induced molten globule state of human acetylcholinesterase: structural and dynamical changes monitored by neutron scattering†
Abstract
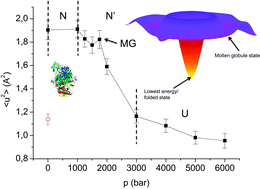
We used small-angle neutron scattering (SANS) to study the effects of high hydrostatic pressure on the structure of human acetylcholinesterase (hAChE). At atmospheric pressure, our SANS results obtained on D11 at ILL (Grenoble, France) give a radius of gyration close to that calculated for a mixture of monomers, dimers and tetramers of the enzyme, suggesting a good agreement between hAChE crystal structure and its conformation in solution. Applying high pressure to the sample we found a global compression of about 11% of the enzyme up to a pressure of 900 bar and then again an extension up to 2.1 kbar indicating unfolding of the tertiary structure due to a molten globule (MG) state. On the other hand, we studied the influence of pressure up to 6 kbar on the dynamics of this enzyme, on the backscattering spectrometer IN13 at ILL. For the first time, we used elastic incoherent neutron scattering (EINS) to probe the differences between hAChE in its folded state (N), its high-pressure induced MG state and its unfolded state (U). Especially around the MG state at 1750 bar we found a significant increase in the dynamics, indicating a partial unfolding. A four-step-model is suggested to describe the changes in the protein.

 Please wait while we load your content...
Please wait while we load your content...