Development of protein seed crystals reinforced with high-strength hydrogels†
Abstract
Neutron macromolecule crystallography can determine the positions of hydrogen atoms in proteins, thereby clarifying the protein structure and function as well as enzyme mechanisms. It requires very large crystals to make up for the low flux of available neutron beams. Some techniques have been developed to obtain large protein crystals. However, problems remain with the handling of the crystal. Here we propose a novel crystallization technique that combines high-strength hydrogel and seeding methods with fatty-acid binding protein and lysozyme. This novel approach enables safe introduction of seeds into pre-equilibrated protein solutions without damage. Consequently, we can grow large single crystals without forming twinned crystals and polycrystals. This technique is expected to expand the scope of important proteins and may lead to an automated system for high-resolution X-ray and neutron crystallography.
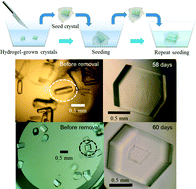
- This article is part of the themed collection: Supramolecular Gels in Crystal Engineering

 Please wait while we load your content...
Please wait while we load your content...