Halogen bonds with coordinative nature: halogen bonding in a S–I+–S iodonium complex†
Abstract
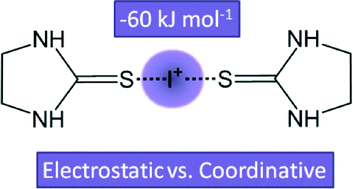
A detailed study of unexpectedly strong iodonium–sulfur halogen bonds in [I(2-imidazolidinethione)2]+ is presented. The interactions are characterized by single-crystal X-ray diffraction, charge density analysis based on QTAIM calculations, mass spectrometry, and NMR spectroscopy. The results, small RIS = 0.7 and high interaction energy of −60 kJ mol−1, support a coordinative nature of the halogen bond between the iodonium ion and the sp2 hybridized sulfur atoms.

 Please wait while we load your content...
Please wait while we load your content...