Structure-directing factors when introducing hydrogen bond functionality to metal–organic frameworks†
Abstract
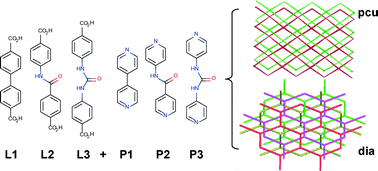
The introduction of H-bond donor/acceptor functionality into metal–organic frameworks (MOFs) can have a beneficial effect on their molecular recognition, uptake selectivity and catalytic properties. The changes in ligand geometry induced by incorporation of functional groups may also affect the topology and composition of the resultant MOFs. Herein, we present a comprehensive study of functional group incorporation into MOFs, linked by either Zn2+ paddlewheel units or monomeric Zn2+ corners, which exhibit pcu and dia topology, respectively. Crystallographic analysis shows that amide groups can be easily incorporated into isoreticular pcu pillared-MOFs, whilst integration of urea units results in materials with dia topology. Molecular simulations allow the examination of hypothetical structures with differing constitutions and topologies, and highlight the influence of the urea units in generating the experimentally observed topologies. Noncovalent interactions between independent nets may be significant structure-directing influences, a finding which has great implications for the design of MOFs containing more complex functional groups.
- This article is part of the themed collection: Metal-Organic Frameworks and Hybrid Materials


 Please wait while we load your content...
Please wait while we load your content...