Palladium-catalyzed dearomative cyclization by a norbornene-mediated sequence: a route to spiroindolenine derivatives†
Abstract
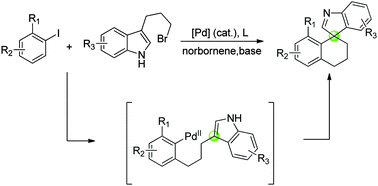
The first palladium-catalyzed dearomative cyclization via a modified Catellani-type C–H functionalization has been realized. The new strategy led to a series of spiroindolenine derivatives bearing an all-carbon quaternary spirocenter from simple aryl halides and substituted indoles.

 Please wait while we load your content...
Please wait while we load your content...