Easy access to constrained peptidomimetics and 2,2-disubstituted azetidines by the unexpected reactivity profile of α-lithiated N-Boc-azetidines†
Abstract
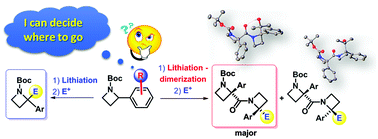
The reactivity profile of lithiated N-Boc-2-arylazetidines has been investigated filling a gap in the chemistry of this class of four-membered heterocycles. Two unexpected and unprecedented results have been observed: an “ortho-effect” accounting for the regioselective functionalization of the azetidine ring, and self-condensation leading to new and interesting azetidine-based peptidomimetics.

 Please wait while we load your content...
Please wait while we load your content...