Mechanism-based design of labile precursors for chromium(i) chemistry†
Abstract
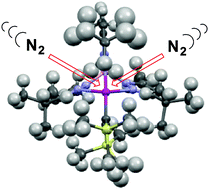
Dinitrogen complexes of the type TpR,RCr–N2–CrTpR,R are not the most labile precursors for Cr(I) chemistry, as they are sterically protected from obligatory associative ligand substitution. A mononuclear alkyne complex – TptBu,MeCr(η2-C2(SiMe3)2) – proved to be much more reactive.

 Please wait while we load your content...
Please wait while we load your content...