Copper-catalyzed oxidative oxyalkylation of enol ethers with α-amino carbonyl compounds and hydroperoxides†
Abstract
A new copper-catalyzed oxidative difunctionalization of enol ethers with α-amino carbonyl compounds and hydroperoxides is developed. This method is experimentally simple while allowing for regioselective access to 2-amino-3,4-dioxy carbonyl compounds in good yields, and represents the first example of alkene oxyalkylation through C(sp3)–H functionalization.
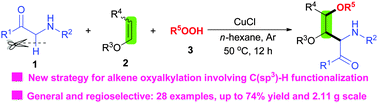

 Please wait while we load your content...
Please wait while we load your content...