Thiophene synthesis via 1,1-carboboration†
Abstract
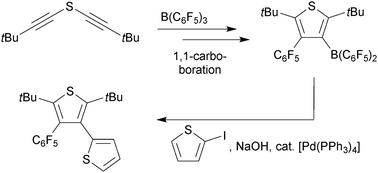
Reaction of bis(tert-butylethynyl)sulfide with the boron Lewis acid reagents X–B(C6F5)2 (X = CH3, Cl, C6F5) in pentane at r.t. gave the respective borylated thiophenes in a sequence of 1,1-carboboration reactions. In contrast, bis(phenylethynyl)sulfide reacted with B(C6F5)3 only in a 2 : 1 molar ratio to give a benzothiophene derivative.

 Please wait while we load your content...
Please wait while we load your content...