Copper-catalyzed oxidative C(sp3)–H/N–H coupling of sulfoximines and amides with simple alkanes via a radical process†
Abstract
A copper-catalyzed oxidative C(sp3)–H/N–H coupling of sulfoximines with simple alkanes was developed. This protocol involved C(sp3)–N bond formation via a radical pathway and tolerated a series of functional groups, such as chloro, methyl and aryl, on the phenyl rings. Apart from sulfoximines, amides, saccharin and aniline also worked well to give the corresponding N-alkylated products.
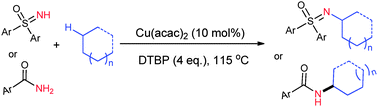

 Please wait while we load your content...
Please wait while we load your content...