Tetrabenzanthanthrenes by mitigation of rearrangements in the planarization of ortho-phenylene hexamers†
Abstract
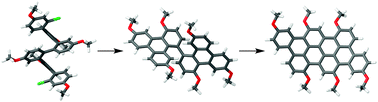
In general, ortho-phenylene hexamers are not good substrates for oxidative planarization because of competing backbone rearrangements. However, by first planarizing the ends, a target tetrabenzanthanthrene has been obtained by oxidation in good yield. DFT calculations suggest that the larger polycyclic aromatic subunits of the preplanarized substrate increase the rate of planarization relative to that of rearrangement. By implication, it may be possible to prepare graphene structures that cannot be made directly from simple polyphenylenes by instead designing precursors with larger polycyclic aromatic moieties. The photophysical properties of the tetrabenzanthanthrene core indicate that it may have promise as a functional chromophore.


 Please wait while we load your content...
Please wait while we load your content...