Cu-catalyzed asymmetric addition of sp2-hybridized zirconium nucleophiles to racemic allyl bromides†
Abstract
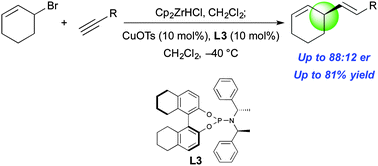
Alkenylzirconium nucleophiles made in situ by the hydrozirconation of terminal alkynes undergo dynamic kinetic asymmetric allylic alkenylation with racemic allyl bromides to give enantioenriched products.

 Please wait while we load your content...
Please wait while we load your content...