Advances in the molecular understanding of biological zinc transport†
Abstract
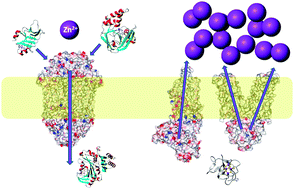
Between 5 and 10% of all proteins of a given organism are estimated to require zinc for function, and hence zinc is essential for almost any given metabolic process. It is therefore of great interest to understand major players and mechanisms that ensure the tight and correct control of zinc distribution and speciation in organisms and their individual cells. Significant progress has been made in recent years regarding 3-dimensional structures and modes of action of zinc sensor proteins, membrane-bound zinc transporters for cellular and sub-cellular uptake and efflux, as well as intracellular binding proteins. This feature article highlights advances in structures, zinc-binding sites and thermodynamics of proteins that are involved in zinc homeostasis and trafficking, including developments in understanding the metal selectivity of proteins.

 Please wait while we load your content...
Please wait while we load your content...