Reversal of enantioselective Friedel–Crafts C3-alkylation of pyrrole by slightly tuning the amide units of N,N′-dioxide ligands†
Abstract
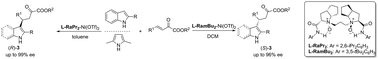
Chiral Ni(II)-complexes of N,N′-dioxides show high catalytic activity and enantioselectivity in catalysing the asymmetric Friedel–Crafts C3-alkylation of 2,5-dimethyl pyrrole to β,γ-unsaturated α-ketoesters. A dramatic reversal of enantioselectivity is realized with ligands derived from the same type of chiral source of L-ramipril, by slightly tuning the amide units.

 Please wait while we load your content...
Please wait while we load your content...