Asymmetric synthesis of α-(1-oxoisoindolin-3-yl)glycine: synthetic and mechanistic challenges†
Abstract
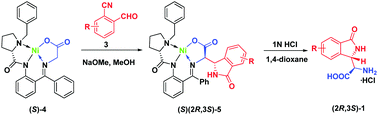
We report herein that the NaOMe-catalyzed reactions between the chiral glycine Schiff base (S)-4 with 2-cyanobenzaldehyde 3a provide for a convenient preparation of the novel α-(1-oxoisoindolin-3-yl)glycine 1 of high pharmaceutical potential. The reactions involve at least eight synthetic steps and can mechanistically be realized only with application of Ni(II) complexes described in this study.

 Please wait while we load your content...
Please wait while we load your content...