Application of the Stern–Volmer equation for studying the spectrofluorimetric quenching reaction of eosin with clindamycin hydrochloride in its pure form and pharmaceutical preparations
Abstract
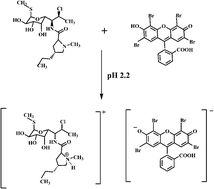
A sensitive, selective, economic, and validated spectrofluorimetric method was developed for the determination of clindamycin hydrochloride in pharmaceutical preparations, depending on the reaction of its tertiary amino group with eosin Y. The proposed method involves quantitative fluorescence quenching of eosin Y measured at 555 nm after excitation at 482 nm upon addition of the studied drug, where the decrease in fluorescence intensity was directly proportional to the concentration of CLD over the concentration range of 0.2–2.0 μg mL−1, with a LOD of 0.13 μg mL−1 and a LOQ of 0.18 μg mL−1. The proposed method was successfully applied to the determination of the studied compound in its dosage forms. The results obtained were in good agreement with those obtained by the official method. Judging from the effects of temperature according to Stern–Volmer plots, the quenching of fluorescence of EY is a static quenching process, resulting from electrostatic attraction and aromatic stacking interactions.

 Please wait while we load your content...
Please wait while we load your content...