Investigating acid-induced structural transitions of lysozyme in an electrospray ionization source†
Abstract
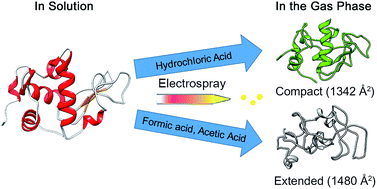
The effect of acids on the structure of lysozyme (Lyz) during electrospray ionization (ESI) was studied by comparing the solution and gas-phase structures of Lyz. Investigation using circular dichroism spectroscopy and small-angle X-ray scattering demonstrated that the folded conformation of Lyz was maintained in pH 2.2 solutions containing different acids. On the other hand, analysis of the charge state distributions and ion mobility (IM) distributions, combined with molecular dynamics simulations, demonstrated that the gas phase structures of Lyz depend on the pKa of the acid used to acidify the protein solution. Formic acid and acetic acid, which are weak acids (pKa > 3.5), induce unfolding of Lyz during ESI, presumably because the undissociated weak acids provide protons to maintain the acidic groups within Lyz protonated and prevent the formation of salt bridges. However, HCl suppressed the formation of the unfolded conformers because the acid is already dissociated in solution, and chloride anions within the ESI droplet can interact with Lyz to reduce the intramolecular electrostatic repulsion. These trends in the IM distributions are observed for all charge states, demonstrating the significance of the acid effect on the structure of Lyz during ESI.

 Please wait while we load your content...
Please wait while we load your content...