Ion mobility coupled to native mass spectrometry as a relevant tool to investigate extremely small ligand-induced conformational changes†
Abstract
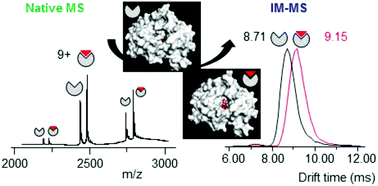
We evaluate the potential of native mass spectrometry (MS) and ion mobility (IM-MS) for the screening of protein : ligand complexes when very subtle conformational changes are involved. As a proof of concept, we investigate the interactions between a peptide deformylase (PDF1B), a promising target for the development of new antibiotics, and three of its specific inhibitors that bind in different modes. First, real-time native MS reveals two types of ligands, both interacting in a 1 : 1 stoichiometry with PDF1B but with different affinities and gas phase stabilities. Conformational IM-MS screening then highlights two very close but significantly distinct ligand-induced conformations with collision cross sections that differ by less than 1%. Real-time IM-MS is used to monitor not only the dynamics of ligand binding to apoPDF1B but also the switching between holo conformations. This study provides additional evidence that the most potent ligands inhibit peptide deformylases through a slow-tight binding mechanism, in agreement with previous structural and enzymology studies. Furthermore, this approach, wherein the characteristics obtained by native MS are combined with IM-MS conformational screening, prove valuable in characterizing extremely subtle dynamic conformational changes induced when ligands bind to protein assemblies. We discuss the promise and limitations of IM-MS in the context of detection of very small conformational changes induced upon ligand binding.
- This article is part of the themed collection: Ion Mobility Mass Spectrometry

 Please wait while we load your content...
Please wait while we load your content...