Azobenzene based multistimuli responsive supramolecular hydrogels†
Abstract
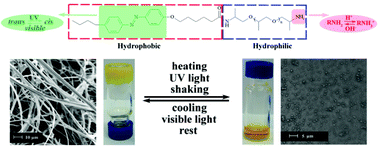
Multistimuli responsive supramolecular aqueous gelators (C4-Azo-C5-D230, C4-Azo-C5-D400, C4-Azo-C5-ED900), composed of alkyl chains, an azobenzene unit, and an amine terminated polyether were prepared. We studied their reversible hydrogelation into three-dimensional entangled supramolecular gels upon changes in temperature, light exposure, pH, and shear. Upon irradiation with UV light, the trans isomer of the C4-Azo-C5-D400 photoisomerized to the cis isomer, which goes to a new steady state between both isomers, resulting in disruption of the gel. Rheological measurements of the hydrogel of C4-Azo-C5-D400 suggested that the non-covalent interactions were disrupted. Likewise, high temperature also caused a reversible disruption to the gel. While the binary mixture of C4-Azo-C5-D400 and water formed gels from a solution under neutral and basic conditions, under the acidic conditions the molecules aggregated and precipitated. After intense shaking of the hydrogel, a solution separated from the gel, resulting in a rapid drop in both modulus and complex viscosity. This photoresponsive gelator can also form lyotropic liquid crystal (LLC) mesophases above 70 °C. Through rational design, multistimuli responsive hydrogelators were successful devised, potentially providing an impetus to the ‘design’ of new gelators through the incorporation of other stimuli responsive features.

 Please wait while we load your content...
Please wait while we load your content...