An electrodeposited lanthanide MOF thin film as a luminescent sensor for carbonate detection in aqueous solution†
Abstract
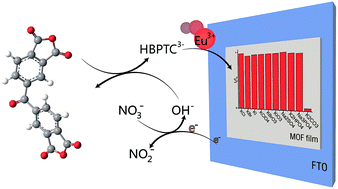
A luminescent lanthanide MOF-based thin film [{[Eu(HBPTC)(H2O)2]·2DMF}n] (BPTC = benzophenone-3,3′,4,4′-tetracarboxylate) was successfully fabricated by electrodeposition in an anhydride system. In this approach, HO− anions will accumulate near the cathode while applying a direct current in nitrate solution, followed by benzophenone-3,3′,4,4′-tetracarboxylic dianhydride (BTDA) hydrolysis and the consequent growth of MOFs directly on an electrode surface. The influence of the concentration of the electrolyte, current density, and conductive salt on the synthesis of MOF films were investigated. We demonstrate that the carboxylic acid ligands can be replaced by the anhydride to fabricate the lanthanide MOF-based thin film by cathodic electrodeposition. Moreover, photoluminescent properties of this film were investigated and the photoluminescent measurement indicated that this luminescent MOF thin film is a highly selective sensor for carbonate in aqueous solution.
- This article is part of the themed collection: 2014 Journal of Materials Chemistry C Hot Articles

 Please wait while we load your content...
Please wait while we load your content...