Asymmetric fused thiophenes for field-effect transistors: crystal structure–film microstructure–transistor performance correlations†
Abstract
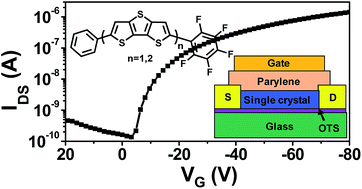
New asymmetric phenyl and perfluorophenyl end-functionalized dithienothiophene (DTT)- and bisdithienothiophene (BDTT)-based fused-thiophene derivatives (FPP-DTT; 1 and FPP-BDTT; 3) were synthesized and characterized for organic thin-film transistor (OTFT) applications. For comparison, symmetric phenyl end-capped dithienothiophene and bisdithienothiophene derivatives DP-DTT (2) and DP-BDTT (4) were also explored in parallel. The crystal structures of all four molecules were determined via single-crystal X-ray diffraction. Asymmetric compounds 1 and 3 exhibit face-to-face π–π stacking, while symmetric 2 and 4 show herringbone stacking. Single-crystal and thin-film transistors based on these four materials were fabricated. For single-crystal transistors, asymmetric FPP-DTT and FPP-BDTT gave high p-channel mobilities of 0.74 and 0.73 cm2 V−1 s−1, respectively, as well as current on/off ratios of ∼105. Symmetric DP-DTT and DP-BDTT gave relatively lower p-channel mobilities of 0.36 and 0.41 cm2 V−1 s−1, respectively. For thin-film transistors, FPP-DTT and DP-DTT films deposited at 25 °C exhibited decent p-channel characteristics with a carrier mobility as high as 0.15 and 0.20 cm2 V−1 s−1, respectively for top-contact/bottom-gate OTFT devices. The device characteristics on various gate dielectrics have been correlated with the film morphologies and microstructures of the corresponding compounds.

 Please wait while we load your content...
Please wait while we load your content...