Relationships between main-chain chirality and photophysical properties in chiral conjugated polymers†
Abstract
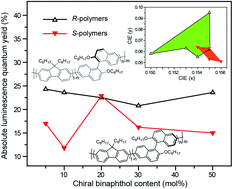
A series of R- and S-binaphthyl-containing polyfluorenes, bearing different contents and types of axial chirality in the main chain, have been synthesized through Suzuki polycondensation to investigate the influence of the covalently incorporated chirality on the photophysical properties of chiral conjugated polymers. The experimental measurements obtained by UV-Vis and photoluminescence (PL) spectroscopy, cyclic voltammetry, and circular dichroism spectroscopy reveal that the chiral copolymers possess high thermostability, high luminescence efficiency, reversible electrochemical properties, and intrachain transferred dichroism. Surprisingly, the R-chiral polymers exhibit better spectral thermostability, stronger suppressing ability upon the β-phase formation of the main-chain, and higher PL quantum efficiency than S-chiral polymers in solid films. The theoretical insights obtained by either ab initio density functional theory (DFT) calculations or molecular dynamics (MD) simulations suggest that these differences probably resulted from the more planar chain conformation of S-chiral polymers, which leads to stronger interchain interaction and an increased tendency to form inefficient and unstable excimers or quenchers. The different effects of enantiomers on the photophysical properties of chiral conjugated polymers may provide an import update in the understanding of chiro-optics.

 Please wait while we load your content...
Please wait while we load your content...