Synthesis and optical properties of iron(iii) complexes of 2-benzylidene-1-indanone derivative thin films†
Abstract
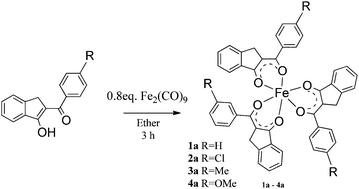
In this work, we propose a different method to synthesize 2-benzylidene-1-indanone derivatives and a new method to obtain iron(III) complexes of 2-benzylidene-1-indanone derivatives, used to prepare semiconducting thin films. The 2-benzylidene-1-indanone derivatives were obtained from the reaction of o-phthalaldehyde with acetophenone in a basic medium and were later complexed with Fe2(CO)9 to form iron(III) complexes from the corresponding redox reaction. One of the iron complexes (4a) was fully characterized by single-crystal X-ray diffraction. Iron(III) complexes of 1-indenol derivative thin films were obtained by thermal evaporation in a high vacuum source. All the samples were grown at room temperature (25 °C) and low deposition rates (0.2 Å s−1) on quartz substrates and (100) single-crystalline silicon (c-Si) wafers. The surface morphology and structure of the deposited films were studied by atomic force microscopy (AFM) and scanning electron microscopy (SEM). Optical absorption studies of the iron(III) complex films were performed in the 100–1150 nm wavelength range. The optical band gap (Eg) of the thin films was determined from the (αhν)1/2vs. hν plots for indirect allowed transitions. The iron(III) complex films show optical activation energies around 2.1 eV, depending on the 1-indenol derivative. From these results, iron complexes of 1-indenol derivatives may prove suitable for photovoltaic or luminescence applications.

 Please wait while we load your content...
Please wait while we load your content...