Development of an ammonia sensor based on silver nanoparticles in a poly-methacrylic acid matrix†
Abstract
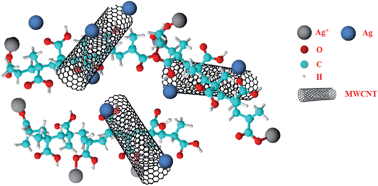
Samples of Ag nanoparticles (AgNPs) in a poly-methacrylic acid (PMA) matrix have been prepared by a photo-induced reduction process. They have been widely characterized by SEM, EDX-mapping, TEM, XRD and XPS measurements. The results have shown that the morphological and microstructural characteristics of AgNPs formed in a PMA matrix depend on the Ag/PMA molar ratio. Thick films of these samples deposited on ceramic substrates have also been prepared. The formation of Ag dendritic structures has been evidenced in samples with a low Ag/PMA ratio, whereas at a higher Ag/PMA ratio the film surface was found to be highly heterogeneous, along with an increase in Ag species in the ionic form. Loading AgNPs/PMA samples with multi-walled carbon nanotubes (MWCNTs) allowed production of conductive films suitable for sensing applications. The samples synthesized were investigated as sensing materials for fabricating ammonia (NH3) gas resistive sensors. The novel sensors developed are active at low temperature, exhibiting fast response/recovery times in a wide range of detection levels from ppm to v/v (%).

 Please wait while we load your content...
Please wait while we load your content...