Local lattice distortions in oxygen deficient Mn-doped ZnO thin films, probed by electron paramagnetic resonance
Abstract
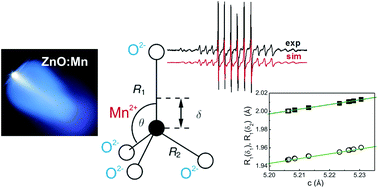
The structure and functional properties of metal oxide films for device applications are largely affected by oxygen vacancies. While the macroscopic relationship between functionality and oxygen supply during growth is easy to access, the local influence of changing oxygen content on the incorporated metal atoms has been rarely investigated. As a model system, we use Mn as a local probe in hetero- and homoepitaxial ZnO thin films for electron paramagnetic resonance (EPR). Mn is expected to be incorporated as Mn2+ in the Zn-lattice site of ZnO films grown in a wide range of oxygen partial pressures. The zero field splitting (ZFS) parameter D depends on the crystallographic c/a ratio of ZnO : Mn lattice constants as it measures the trigonal distortion of oxygen tetrahedra at the Zn2+ site with respect to the Mn2+ site. The ZFS parameter D correlates linearly with displacement of Mn2+ ions along the c-axis in the MnO4 tetrahedra and the corresponding bond lengths between the Mn2+ ions and the axial oxygen ion.

 Please wait while we load your content...
Please wait while we load your content...