Substituent effect on the crystal packing and electronic coupling of tetrabenzocoronenes: a structure–property correlation†
Abstract
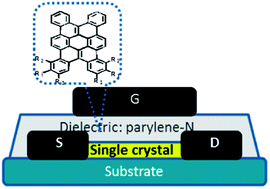
Tetrabenzo[a,d,j,m]coronene (TBC) is a contorted polyaromatic molecule which shows near co-facial π–π stacking in the crystalline state and the high electronic coupling resulting from the packing renders it a potential candidate as a transistor material. Substitution at the periphery perturbs the packing due to steric as well as dipolar interactions and thus changes the electronic coupling between neighbouring molecules. In the light of the high sensitivity of charge mobility toward electronic coupling, a new series of TBC derivatives with substituents at 1-, 2-, 3-, 6-, 7-, 8-positions were designed, synthesized, and characterized. Needle-like single crystals were prepared using the physical vapor transport (PVT) method for these unsymmetrically substituted derivatives and were used for crystal structure analyses as well as the single crystal field-effect transistor (SCFET) device fabrication. The derivatives with fluoro-containing substituents exhibit anti-parallel cofacial or slightly shifted π–π stacking, whereas those with bulky alkyl substituents show skewed and more significantly shifted π–π stacking. A systematic comparison of the crystal packings and the calculated electronic couplings/charge mobilities with the measured SCFET mobilities shows a rough correlation and sheds light on the origin of the large hole-mobility of the SCFET with hexa-fluorinated TBC as the channel material.

 Please wait while we load your content...
Please wait while we load your content...