Thermoelectric properties of a single graphene sheet and its derivatives†
Abstract
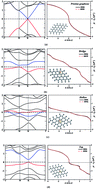
The thermoelectric properties of pristine graphene and H2S adsorbed onto bridge, hollow and top sites of a graphene sheet are investigated using the semi-classical Boltzmann transport theory. The average values of electrical conductivity, thermal conductivity, Seebeck coefficient, figure of merit (ZT) and the average value of the power factor (Pav) are reported and discussed in detail. While pristine graphene is a zero band gap semiconductor, adsorption of H2S onto the bridge site opens up a direct energy gap of about 0.04 eV, adsorption of a H2S molecule onto the top site opens up a gap of 0.3 eV, and adsorption of H2S onto the hollow site makes it metallic. The investigation of ZT and power factor values suggests that a top-site configuration could be a potential candidate for thermoelectric applications in the range 300–600 K.

 Please wait while we load your content...
Please wait while we load your content...