Synthesis and optical reactivity of 6,13-α-diketoprecursors of 2,3,9,10-tetraalkylpentacenes in solution, films and crystals†
Abstract
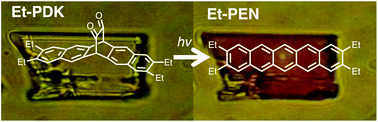
Tetraalkylpentacenes having alkyl chains at 2,3,9,10-positions (Et-PEN, Pr-PEN and Hex-PEN) were prepared from their precursors Et-PDK, Pr-PDK and Hex-PDK, respectively. Photoreactions proceeded both in solutions, thin-films, and crystals, thus the properties of Et-PDK in films can be studied despite the instability of the pentacenes in solution. Et-PEN showed significantly different aggregation-nature compared with the parent pentacene. The hole mobilities of Et-PEN and Pr-PEN in films were 3.4 × 10−6 and 8.1 × 10−7 cm2 V−1 s−1, respectively, determined by space-charge-limited current measurement, comparable with the order 10−6 cm2 V−1 s−1 of the electron mobility of Alq3.

 Please wait while we load your content...
Please wait while we load your content...