Carborane–β-cyclodextrin complexes as a supramolecular connector for bioactive surfaces†
Abstract
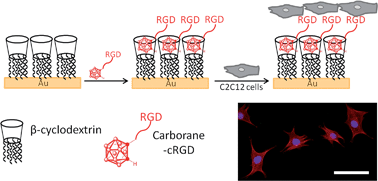
Supramolecular chemistry provides an attractive entry to generate dynamic and well-controlled bioactive surfaces. Novel host–guest systems are urgently needed to provide a broader affinity and applicability portfolio. A synthetic strategy to carborane–peptide bioconjugates was therefore developed to provide an entry to monovalent supramolecular functionalization of β-cyclodextrin coated surfaces. The β-cyclodextrin·carborane–cRGD surfaces are formed efficiently and with high affinity as demonstrated by IR-RAS, WCA, and QCM-D, compare favourable to existing bio-active host–guest surface assemblies, and display an efficient bioactivity, as illustrated by a strong functional effect of the supramolecular system on the cell adhesion and spreading properties. Cells seeded on the supramolecular surface displaying bioactive peptide epitopes exhibited a more elongated morphology, focal adhesions, and stronger cell adhesion compared to control surfaces. This highlights the macroscopic functionality of the novel supramolecular immobilization strategy.

 Please wait while we load your content...
Please wait while we load your content...