In vivo assessment of grafted cortical neural progenitor cells and host response to functionalized self-assembling peptide hydrogels and the implications for tissue repair
Abstract
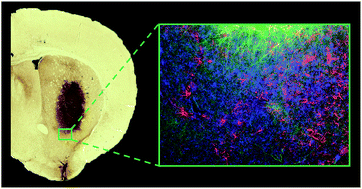
Tissue specific scaffolds formed from minimalist N-fluorenylmethyloxycarbonyl self-assembling peptides (Fmoc-SAPs) have emerged as promising biomaterials due to their ease of synthesis and capacity to self-assemble via simple, non-covalent interactions into complex nanofibrous hydrogels. However, concerns remain over their biocompatibility and cytotoxicity for in vivo applications. Here, we demonstrate that these Fmoc-SAPs are biocompatible in vivo and well suited as a delivery vehicle for cell transplantation. In order to determine the effect of tissue specific parameters, we designed three Fmoc-SAPs containing varying bioactive peptide sequences derived from extracellular matrix proteins, laminin and fibronectin. Fmoc-SAPs delivering cortical neural progenitor cells into the mouse brain display a limited foreign body response, effective functionalization and low cytotoxicity for at least 28 days. These results highlight the suitability of Fmoc-SAPs for improved neural tissue repair through the support of grafted cells and adjacent host parenchyma. Overall, we illustrate that Fmoc-SAPs are easily engineered materials for use as a tool in cell transplantation, where biocompatibility is key to promoting cell survival, enhancing the graft–host interface and attenuation of the inflammatory response for improved tissue repair outcomes.

 Please wait while we load your content...
Please wait while we load your content...