Bioinspired silica as drug delivery systems and their biocompatibility†
Abstract
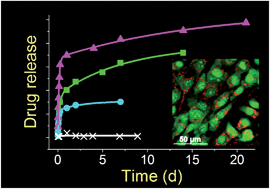
Silica nanoparticles have been shown to have great potential as drug delivery systems (DDS), however, their fabrication often involves harsh chemicals and energy intensive laborious methods. This work details the employment of a bioinspired “green” method for the controlled synthesis of silica, use of the products to entrap and release drug molecules and their cytotoxicity in order to develop novel DDS. Bioinspired silica synthesis occurs at pH 7, room temperature and in less than 5 minutes, resulting in a rapid, cheaper and greener route. Drugs were loaded into silica during the silica formation, thus allowing a one step and one pot method for simultaneous silica synthesis and drug loading. We established that the drug release profile can be modulated by synthetic parameters, which can allow design of tailored DDS. A systematic investigation using a two level factorial design was adopted in order to identify the key synthetic parameters and quantify their effects on silica formation, drug loading and drug release. The observation that these new DDS are considerably less cytotoxic than their current counterparts, and exhibit additional benefits such as green synthesis and ease of functionalization, strengthens the argument for their future use in DDS and other biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...