Self-assembly and pH response of electroactive liquid core–tetra(aniline) shell microcapsules†
Abstract
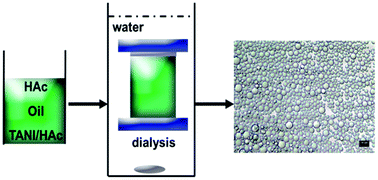
We developed a simple one-pot method to fabricate electroactive tetra(aniline)-based microcapsules, which exhibit a wide range of pH response by self-assembly. In this procedure, the microcapsules are prepared by the facile dialysis-induced self-assembly of tetra(aniline), TANI, doped with acetic acid (HAc), onto the oil-in-water droplet interface. The chemical structure of the shell is characterized by FT-IR and UV-Vis spectroscopy. The electrochemical properties, self-assembly and pH response of microcapsule dispersions are further investigated by cyclic voltammetry, optical and electron microscopy, dynamic light scattering and aqueous electrophoresis. The size of the microcapsules increases gradually from 1.25 μm to 2.93 μm when the concentration of HAc changes from 12 M to 14 M and higher (to become glacial acetic acid). Furthermore, we show that aggregation and stability of the microcapsules can be controlled through changes in pH. This strategy appears to be a general scheme for producing other oligo(aniline)-based microcapsules.

 Please wait while we load your content...
Please wait while we load your content...