Self-assembled dipeptide nanotubes constituted by flexible β-phenylalanine and conformationally constrained α,β-dehydrophenylalanine residues as drug delivery system†
Abstract
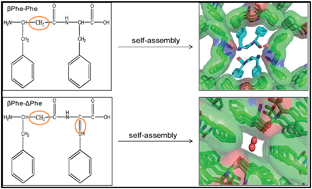
Peptide based self assembled nanostructures have attracted growing interest in recent years due to their numerous potential applications particularly in biomedical sciences. Di-peptide Phe–Phe was shown previously to self-assemble into nanotube like structures. In this work, we studied the affect of peptide backbone length and conformational flexibility on the self assembly process by using two dipeptides based on the Phe–Phe backbone (βPhe–Phe and βPhe–ΔPhe): one containing a flexible βPhe amino acid, and the other containing both a flexible βPhe as well as a backbone constraining ΔPhe (α,β-dehydrophenylalanine) amino acid. Electron microscopy and X-ray diffraction experiments revealed that these new di-peptides can self-assemble into nanotubes having different properties than the native Phe–Phe nanotubes. These nanotubes were stable over a broad range of temperatures and the introduction of non-natural amino acids provided them with stability against the action of nonspecific proteases. Moreover, these dipeptides showed no cytotoxicity towards HeLa and L929 cells, and were able to encapsulate small drug molecules. We further showed that anticancerous drug mitoxantrone was more efficient in killing HeLa and B6F10 cells when entrapped in nanotubes as compared to free mitoxantrone. Therefore, these β-phenylalanine and α,β-dehydrophenylalanine containing dipeptide nanotubes may be useful in the development of biocompatible and proteolytically stable drug delivery vehicles.

 Please wait while we load your content...
Please wait while we load your content...