The dissolution and biological effects of silver nanoparticles in biological media
Abstract
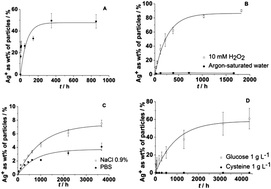
Silver ions and silver nanoparticles have a well-known biological effect that typically occurs in biological or environmental media of complex composition. Silver nanoparticles release silver ions if oxidizing species like molecular oxygen or hydrogen peroxide are present. The presence of glucose as a model for reducing sugars has only a small effect on the dissolution rate. In the presence of chloride ions, precipitation of silver chloride nanoparticles occurs. At physiological salt concentrations, no precipitation of silver phosphate occurs as the precipitation of silver chloride always occurs first. If the surface of a silver nanoparticle is passivated by cysteine, the dissolution is quantitatively inhibited. Upon immersion of silver nanoparticles in pure water for 8 months, leading to about 50% dissolution, no change in the surface was observed by transmission electron microscopy. A model for the dissolution was derived from immersion and dissolution experiments in different media and from high-resolution transmission electron microscopy. A literature survey on the available data on the dissolution of silver nanoparticles showed that only qualitative trends can be identified as the nature of the nanoparticles and of the immersion medium are practically never comparable. The dissolution effects were confirmed by cell culture experiments (human mesenchymal stem cells and neutrophil granulocytes) where silver nanoparticles that were stored under argon had a clearly lower cytotoxicity than those stored under air. They also led to a less formation of reactive oxygen species (ROS). This underscores that silver ions are the toxic species.

 Please wait while we load your content...
Please wait while we load your content...