Chemical characterisation and fabrication of chitosan–silica hybrid scaffolds with 3-glycidoxypropyl trimethoxysilane†
Abstract
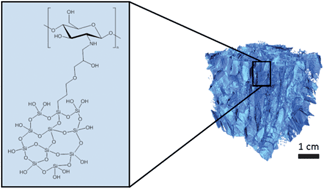
Chitosan has been explored as a potential component of biomaterials and scaffolds for many tissue engineering applications. Hybrid materials, where organic and inorganic networks interpenetrate at the molecular level, have been a particular focus of interest using 3-glycidoxypropyl trimethoxysilane (GPTMS) as a covalent crosslinker between the networks in a sol–gel process. GPTMS contains both an epoxide ring that can undergo a ring opening reaction with the primary amine of chitosan and a trimethoxysilane group that can co-condense with silica precursors to form a silica network. While many researchers have exploited this ring-opening reaction, it is not yet fully understood and thus the final product is still a matter of some dispute. Here, a detailed study of the reaction of GPTMS with chitosan under different pH conditions was carried out using a combination of solution state and solid state MAS NMR techniques. The reaction of GPTMS with chitosan at the primary amine to form a secondary amine was confirmed and the rate was found to increase at lower pH. However, a side-reaction was identified between GPTMS and water producing a diol species. The relative amounts of diol and chitosan–GPTMS species were 80 and 20% respectively and this ratio did not vary with pH. The functionalisation pH had an effect on the mechanical properties of 65 wt% organic monoliths where the properties of the organic component became more dominant. Scaffolds were fabricated by freeze drying and had pore diameters in excess of 140 μm, and tailorable by altering freezing temperature, which were suitable for tissue engineering applications. In both monoliths and scaffolds, increasing the organic content disrupted the inorganic network, leading to an increase in silica dissolution in SBF. However, the dissolution of silica and chitosan was congruent up to 4 weeks in SBF, illustrating the true hybrid nature resulting from covalent bonding between the networks.

 Please wait while we load your content...
Please wait while we load your content...