Enhancing visible-light photoelectrochemical water splitting through transition-metal doped TiO2 nanorod arrays†
Abstract
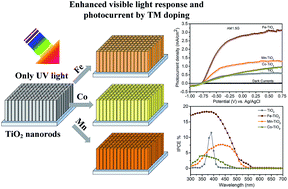
Extending the photoresponse from the ultraviolet (UV) to the visible light region, while maintaining a high photocatalytic activity has been an important challenge for TiO2. We demonstrate the use of transition-metal doping treatment as a facile and effective strategy to substantially improve the performance of TiO2 nanorods in the visible light region for photoelectrochemical (PEC) water splitting. The effect of Fe, Mn and Co as dopants on the PEC performance of the TiO2 nanorods was investigated, wherein the Fe doping is the most effective route to enhance the photoactivity of TiO2. The photocurrent density of Fe–TiO2 sample significantly increases with bias voltage and reaches 2.92 mA cm−2 at 0.25 V vs. Ag/AgCl, which is five times higher than that of the undoped TiO2. Even under visible light illumination (>420 nm), the photocurrent density of Fe–TiO2 is as high as 0.96 mA cm−2 at 0.25 V vs. Ag/AgCl. Incident-photon-to-current-conversion (IPCE) efficiency (up to ∼18%) measurements reveal that the Fe–TiO2 nanorod sample significantly improve the photoresponse not only in the UV region, but also in the visible light region. Fe doping not only enhances the visible light absorption of TiO2 nanorods by creating impurity states near the conduction band, but also obviously increases carrier density of TiO2, leading to effective carrier separation and transportation and relatively long electron lifetime. Because of their relatively high photocatalytic activity, the Fe–TiO2 nanorods can serve as a promising candidate in various areas, such as solar water splitting, dye-sensitized solar cells, and photocatalysis.

 Please wait while we load your content...
Please wait while we load your content...