d-Glucopyranose-modified compound of Ruddlesden–Popper phases H2CaTa2O7: characterization and intercalation with Ag†
Abstract
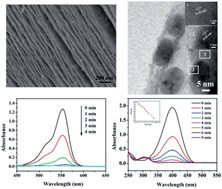
D-Glucopyranose rings have been successfully coordinated onto the interlayer surface of a Ruddlesden–Popper-type layered perovskite, H2CaTa2O7, by a reaction with the precursor of n-decoxyl derivative of H2CaTa2O7. The interlayer distance of D-glucopyranose derivative of H2CaTa2O7 was decreased to 19.135(5) Å compared to 35.026(0) Å for n-decoxyl derivative of H2CaTa2O7. IR and solid-state 13C CP/MAS NMR spectra indicated that the oxyalkyl chains of the precursor were replaced by glucopyranose rings. To extend inorganic–organic hybrids, the H2CaTa2O7 modified was further treated with [Ag(NH3)2]+ solution. Ag nanoparticles are deposited on the interlayer of H2CaTa2O7, and the interlayer distance of the samples is changed into 10.816(6) Å. The as-prepared nanohybrids (Ag/H2CaTa2O7) exhibit an excellent catalytic property for the catalytic reduction of both rhodamine B (RhB) and 4-nitrophenol (4-NP) by NaBH4 aqueous solution; therefore, it is a practical and efficient nanocatalyst.

 Please wait while we load your content...
Please wait while we load your content...