Synthesis and characterization of Mn-based composite oxides with enhanced electrocatalytic activity for oxygen reduction
Abstract
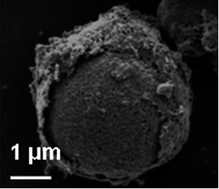
Implementation of non-precious electrocatalysts towards the oxygen reduction reaction (ORR) is the central focus for fulfilling cost-affordable and high-performance fuel cells and metal/air batteries. Herein, we report a modified solvothermal approach for the preparation of composite carbonates between Mn and X (X = Co, Ni and Fe). Upon post-annealing treatment, the aforementioned carbonates are transformed into the corresponding micro/nano hierarchical structured mixed oxides with increased porosity. It is found that onion-like core–shell architectures appear in the Mn–Co and Mn–Ni systems because of volume shrinkage arising from the generation of chemical-level mixed oxides, while a less porous structure occurs in the Mn–Fe system with the formation of a physical-level mixture. The ORR activity of the prepared composite oxides in alkaline media is investigated by voltammetry under different hydrodynamic conditions. An enhanced ORR activity is observed in the Mn–Co mixed oxide, which is rationalized in terms of the unique microstructure and crystallographic phase. The present study suggests that proper mixing is an effective method for activating the ORR activity of manganese oxides, which is beneficial for developing non-precious electrocatalysts for fuel cells and metal/air batteries.

 Please wait while we load your content...
Please wait while we load your content...