Influence of carbon pore size on the discharge capacity of Li–O2 batteries†
Abstract
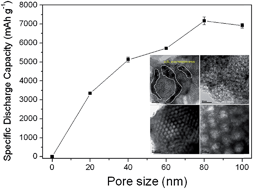
Porous carbon materials play key roles in rechargeable Li–O2 batteries as oxygen diffusion media and sites for reversible electrode reactions. Despite tremendous efforts in the synthesis of various porous carbon materials, the influence of carbon materials on cell capacity remains unclear. Based on our study of eight different carbon electrode materials with various pore sizes and pore volumes in Li–O2 batteries, we found that the initial discharge capacity was hardly affected by the surface area or pore volume. Instead, it was directly correlated with the pore sizes. To further verify this finding, meso- and macro-porous carbon materials with pore sizes in the range of 20 to 100 nm were prepared using spherical silica as a template. The results clearly showed that the cell capacity increases with the increase of pore size and eventually reached its maximum at 7169 mA h g−1 at a pore size of 80 nm. A physical model proposed to illustrate the influence of carbon pore size on cell capacity is the formation of a monolayer of Li2O2 with a thickness of 7.8 nm inside the carbon pores during the discharge process which limits the diffusion of incoming oxygen at smaller pore size (<80 nm).

 Please wait while we load your content...
Please wait while we load your content...