A guest-dependent thermochromic feature in a metal–organic framework and its thin film on different supports†
Abstract
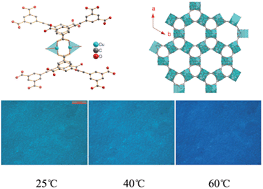
A three-dimensional NbO-type metal–organic framework (MOF) is composed of paddle-wheel-type dinuclear Cu2 secondary units and 1,1′-ethynebenzene-3,3′,5,5′-tetracarboxylate (EBTC4−) linkers. Two types of nanometer-sized cavities are formed in this framework with ca. 8.5 Å in diameter for the small one and dimensions of ca. 8.5 × 8.5 × 21.5 Å for the larger and irregular elongated cavity. The guest molecules, H2O, DMF and DMSO, occupy the cavities of the as-prepared MOF crystal (labeled as MOF 1). MOF 2 was obtained by the guest-exchange approach using CH3OH, and the H2O and CH3OH molecules reside in the cavities of 2. Two MOFs show greenish-turquoise color at ambient temperature due to the d–d transition of Cu2+ ions in the framework, and the reversible thermochromic behavior owing to the change of the coordination environment of Cu2+ ions with varying temperatures. The films of 1 and 2 were fabricated on the α-Al2O3 and SiO2 supports by the seeded growth method, displaying similar reversible thermochromic behavior to the corresponding MOFs. This study suggested the possibility of novel thermochromic materials in the rational design of MOFs.

 Please wait while we load your content...
Please wait while we load your content...