Amphiphilic poly(acrylonitrile)-co-poly(2-dimethylamino)ethyl methacrylate conetwork-based anion exchange membrane for water desalination†
Abstract
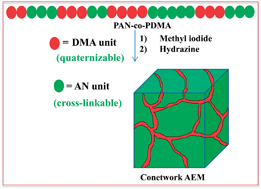
We report the preparation of a novel cross-linked anion exchange membrane (AEM) from poly(acrylonitrile)-co-poly(2-dimethylamino)ethyl methacrylate (PAN-co-PDMA) copolymer by quaternizing the DMA moieties followed by cross-linking of –CN groups. This process avoids the use of the carcinogenic reagent, chloromethyl ether (CME) which is widely employed for the chloromethylation reaction in the preparation of AEMs. Key parameters were evaluated via electrodialysis (ED) during water purification and revealed that a copolymer containing 28 wt% PDMA possesses all the required properties of a high performance AEM, such as, reasonable water uptake (21%), good ion-exchange capacity (IEC = 1.30 m eq g−1), high ionic conductivity (Km = 2.22 mS cm−1), a high transport number (t− = 0.88) and good mechanical properties (9.2 MPa of tensile stress at break and 2.4 Kg cm−2 of burst strength in the water wet state). The AEM was directly tested in an ED unit in the recirculation mode by varying the concentration of the NaCl feed and the applied potential, showing a current efficiency as high as 80%. Such combination of physical and electrochemical properties of the membrane is attributed to its co-continuous morphology and its low degree of phase separation as confirmed by swelling, differential scanning calorimetry, the homogeneous distribution of oppositely charged dye and by the transparency of the membrane in both the dry and swelled states.

 Please wait while we load your content...
Please wait while we load your content...